Poster
Click to view the PDF version.
Abstract
Poster presented at ASPEN25 conference.
You can view the original article here (on page 53):
aspenjournals.onlinelibrary.wiley.comAuthors
Osman Mohamed Elfadil, MBBS1; Edel Keaveney, PhD2; Adele Pattinson, RDN1; Danelle Johnson, MS, RDN1; Rachael Connolly, BSc.2; SuhenaPatel, MBBS1; Yash Patel, MBBS1; Ryan Hurt, MD, PhD1; Manpreet Mundi, MD1
1 Mayo Clinic, Rochester, MN; 2 Rockfield MD, Galway
Background
Patients on home enteral nutrition (HEN), many of whom are mobile, can experience significant hardships and reduced quality of life (QoL) due to limitations on mobility, on top of burdens due to underlying disease processes. Improving mobility while feeding could reduce burdens associated with HEN and potentially improve QoL. This prospective cohort study aims to evaluate participants’ perspectives on their mobility, ease of performing physical activities while feeding, and QoL following the use of a novel enteral feeding system (EFS).
Methods
A prospective single‐center study was conducted to evaluate a novel EFS, which is an FDA‐cleared elastomeric system (Mobility+®) that consists of a lightweight feeding pouch (reservoir for 500 mL feed), a filling set (used in conjunction with a syringe to fill EFS) and a feeding set to deliver EN formula to an extension set/feeding tube with an ISO 80369‐3 compatible connector.
Adult HEN‐dependent patients were recruited by invitation to use the study EFS for a minimum of 2 feeds a day for 14 days, preceded by a familiarization period of 5‐7 days.
Participant perspectives on how they rated performing typical daily activities while feeding (e.g., moving, traveling, socializing) and feeding system parameters were evaluated using HEN‐expert validated questionnaires:
- ease of use
- portability
- noise
- discretion
- performance
A score was given for each rating from 1 to 5, with 5 being the most positive response. An overall score was calculated and averaged for the cohort. Participants were followed up during the familiarization period.
On days 7 and 14, additional telephone interviews were conducted regardingcompliance, enteral feed intake, participant perspectives on study EFS vs. current system, and other measures. We excluded those with reducedfunctional capacity due to their underlying disease(s).
Results
Seventeen participants completed the study (mean age 63.8 ± 12 years; 70.6% male).
Participants used various feeding systems, including gravity, bolus method, and pump, with the majority (82.4%) having a G‐tube placed (Table 1).
Sixteen (94.1%) patients achieved use of study EFS for at least two feeds a day (and majority of daily EN calories) for all study days (Table 2).
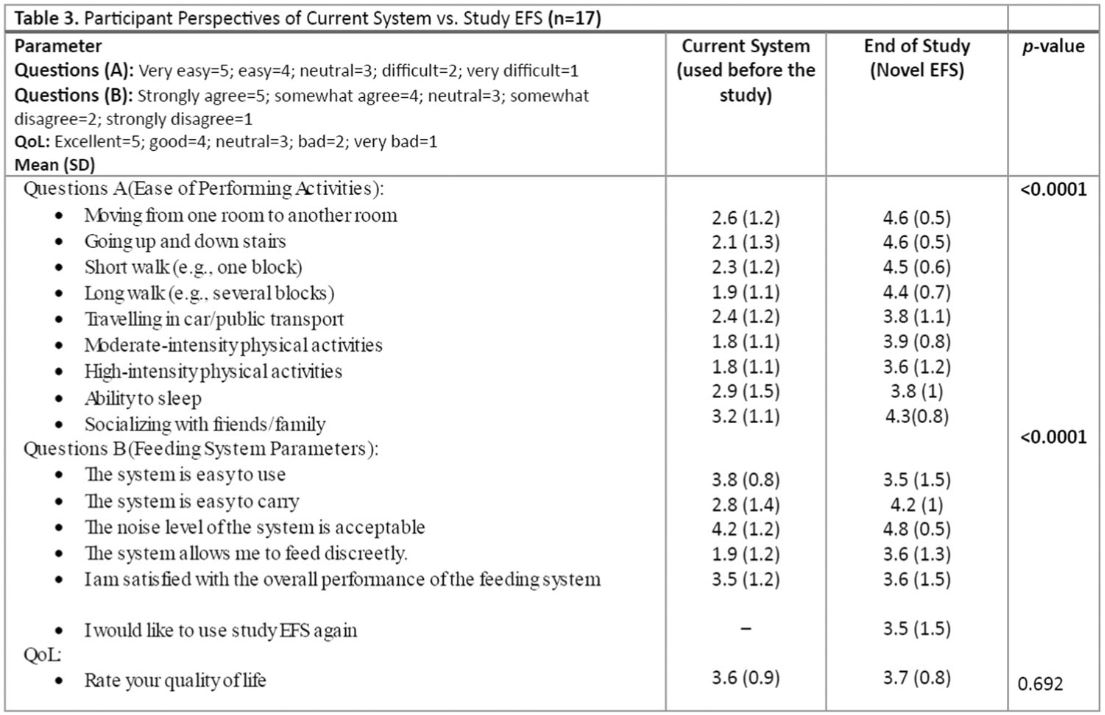
The ratings for the ability to perform various activities using study EFS were significantly different compared to those of the systems used before the study. An improvement in ratings was noted for the ease of performing common daily activities, including moving between rooms or on stairs, taking short and long walks, traveling by car or public transport, engaging in moderate‐ to high‐intensity activities, sleeping, and socializing with family and friends, between the time point before enrolment and end of study (day 14) (p‐value < 0.0001) (Table 3).
Ratings of feeding system parameters were significantly different between systems used before the study and the study EFS (p < 0.0001) (Table 3), with the largest increases in positive ratings noted in relation to easiness to carry, noise level, and ability to feed discreetly.
Ratings for overall satisfaction with the performance of study EFS did not differ from the ratings for the systems used before the study, with participants reporting that the main influencing factors were the length of time and the effort needed to fill study EFS. No difference was noted in the QoL rating.
Conclusion
The studied EFS is safe and effective as an enteral feeding modality that provides an alternative option for HEN recipients.
Participants reported a significant positive impact of study EFS on their activities of daily living.
Although the overall QoL rating remained the same, improvements in mobility, discretion, and ease of carrying— aspects of QoL—were associated with the use of study EFS.
Table 1
Baseline Demographics and Clinical Characteristics.
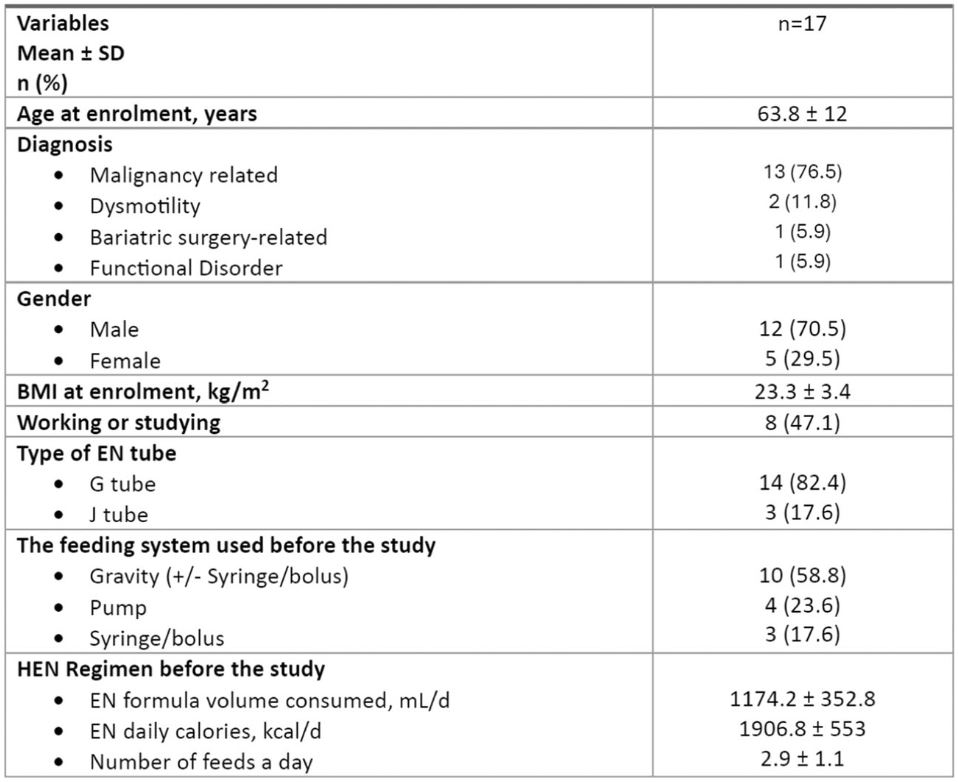
Table 2
Safety and Effectiveness.
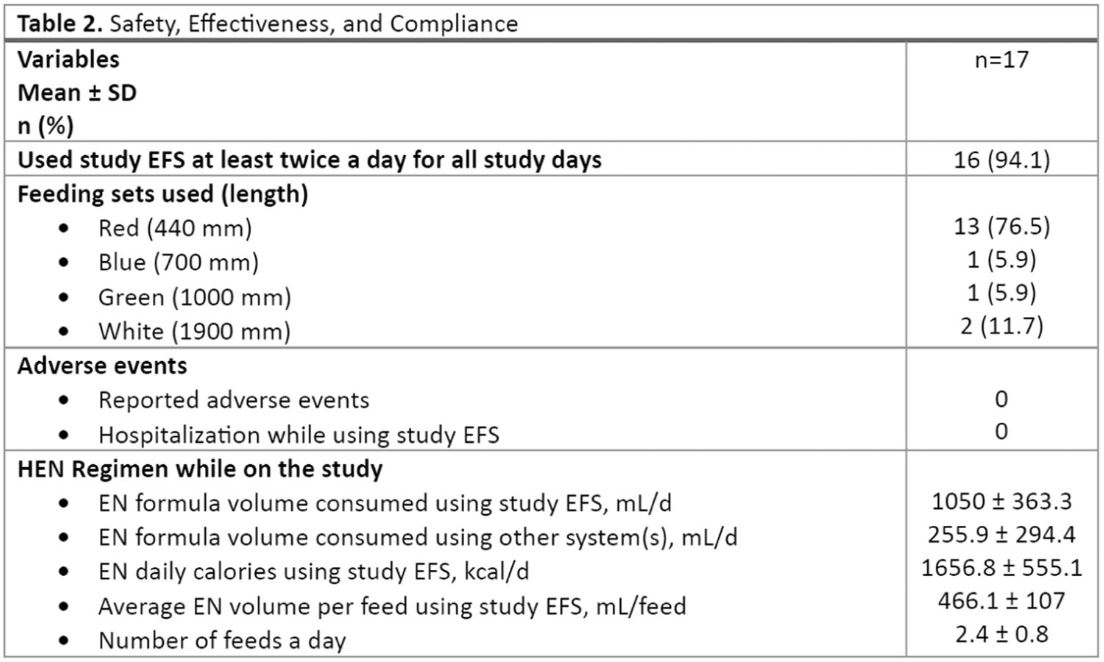
Table 3
Usability and Impact of the Study EFS.